By JONEL ALECCIA
AP Health Writer
As cases of potentially deadly botulism in babies who drank ByHeart infant formula continue to grow, state officials say they are still finding the recalled product on some store shelves.
Meanwhile the company reported late Wednesday that laboratory tests confirmed that some samples of formula were contaminated with the type of bacteria that has sickened more than 30 babies in the outbreak.

Tests by an independent food safety laboratory found Clostridium botulinum, a bacterium that produces toxins that can lead to potentially life threatening illness in babies younger than 1, the company said on its website. ByHeart officials said they notified the U.S. Food and Drug Administration of the findings but did not specify how many samples were tested or how many were positive.
“We are working to investigate the facts, conduct ongoing testing to identify the source, and ensure this does not happen to families again,” ByHeart said on its website.
The FDA did not immediately respond to questions about the findings.
The lab results come as investigators in at least three states found ByHeart formula still for sale even after the New York-based company recalled all products nationwide, officials told The Associated Press.
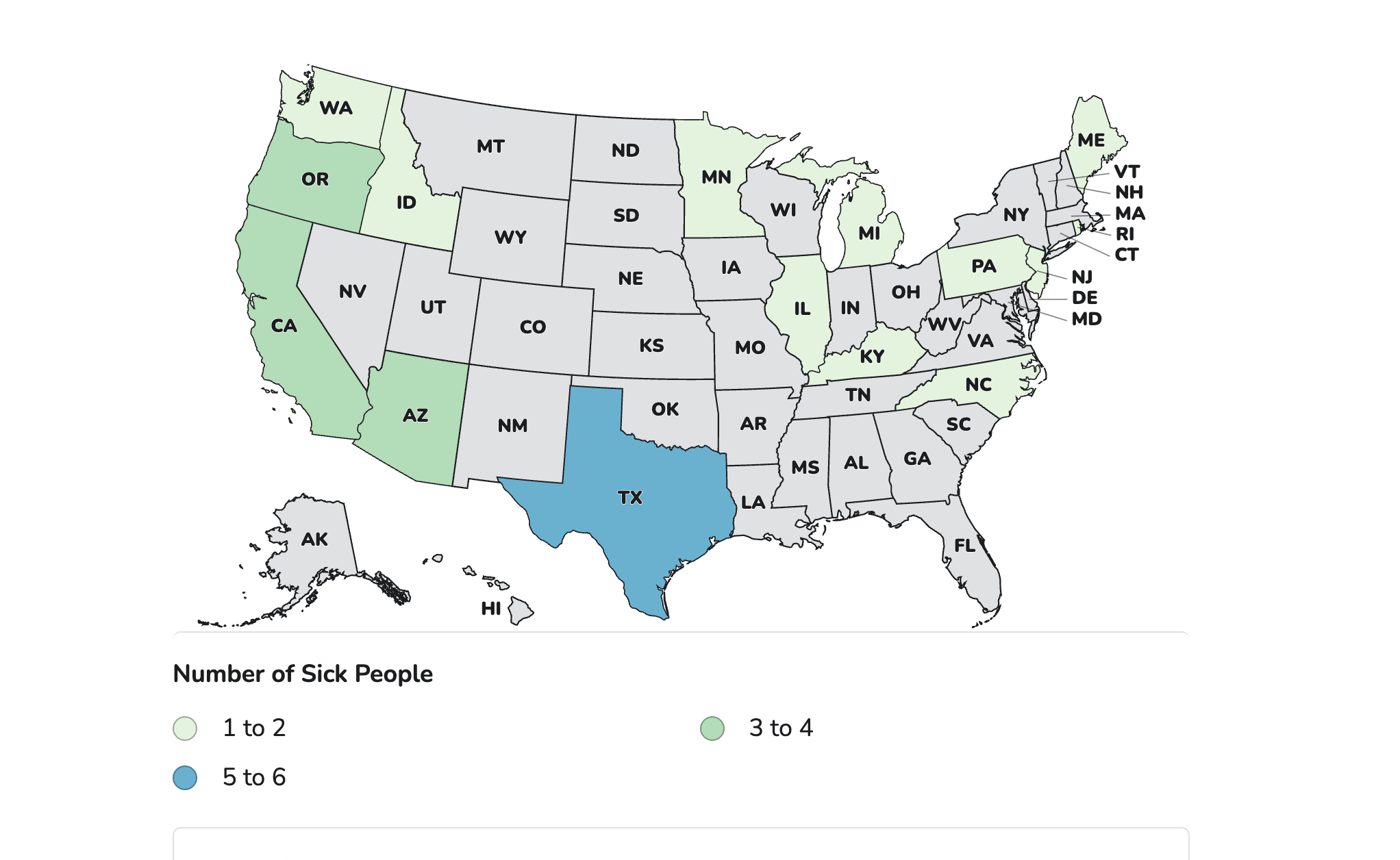
At least 31 babies in 15 states who drank ByHeart formula have been hospitalized and treated for infantile botulism since August, federal health officials said Wednesday. They range in age from about 2 weeks to about 6 months, with the most recent case reported on Nov. 13.
No deaths have been reported.
In Oregon, nine of more than 150 stores checked still had the formula on shelves this week, a state agriculture official said. In Minnesota, investigators conducted 119 checks between Nov. 13 and Nov. 17 and removed recalled products from sale at four sites, an agriculture department official said. An Arizona health official also said they found the product available.
Businesses and consumers should remain alert, Minnesota officials said in a statement. “No affected product should be sold or consumed,” they wrote.
Investigators with the U.S. Food and Drug Administration conducted inspections at ByHeart manufacturing plants in Allerton, Iowa, and Portland, Oregon. No results from the inspections have been reported.
California officials previously confirmed the germ that can lead to illness in an open can of ByHeart formula fed to a baby who fell ill.
Infant botulism, which can cause paralysis and death, is caused by a type of bacteria that forms spores that germinate in a baby's gut and produce a toxin.
Symptoms can take up to 30 days to develop and include constipation, poor feeding, a weak cry, drooping eyelids or a flat facial expression. Babies can develop weakness in their limbs and head and may feel “floppy.” They can have trouble swallowing or breathing.
ByHeart had been manufacturing about 200,000 cans of formula per month. It was sold online or at retail stores such as Target and Walmart. A Walmart spokesperson said the company swiftly issued a restriction that prevented sale of the formula, removed the product from stores and notified consumers who had bought it. Customers can visit any store for a refund of the formula, which sold for about $42 per can.
Federal and state health officials are concerned that some parents and caregivers may still have ByHeart products in their homes. They are advising consumers to stop using the product — including formula in cans and any single-serve sticks. They also suggest marking it “DO NOT USE” and keeping it for at least a month in case a baby develops symptoms. In that case, the formula would need to be tested.
The California health department operates the Infant Botulism Treatment and Prevention Program, which tracks cases and distributes treatment for the disease. Officials there have launched a public hotline at 833-398-2022, which is staffed with health officials from 7 a.m. to 11 p.m. Pacific Standard Time.
The new hotline was created after calls from hundreds of parents and caregivers flooded a different, longstanding hotline for doctors to discuss suspected infant botulism cases, officials said.
___
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education and the Robert Wood Johnson Foundation. The AP is solely responsible for all content.
-------------
More than 30 babies in 15 states have been sickened in a growing outbreak of infant botulism linked to recalled ByHeart infant formula, federal health officials said Wednesday.
The U.S. Centers for Disease Control and Prevention reported 31 cases of confirmed or suspected illness in babies who consumed ByHeart formula since August. The most recent case was reported on Nov. 13.
No deaths have been reported in the outbreak, which was announced Nov. 8.
“Do not use any ByHeart Whole Nutrition infant formula,” the CDC said.
ByHeart, a New York-based manufacturer of organic infant formula, has recalled all its products sold in the U.S. The company, which accounts for about 1% of the U.S. infant formula market, had been selling about 200,000 cans of the product each month.
It can take up to 30 days for symptoms of infantile botulism to develop, medical experts said.
Here’s what to know about the outbreak and infant botulism.
The outbreak begins
The outbreak has sickened babies aged about 2 weeks to about 6 months, the FDA said. All the infants were hospitalized after consuming ByHeart powdered formula.
California officials confirmed that a sample from an open can of ByHeart baby formula fed to an infant who fell ill contained the type of bacteria that can lead to illness.
ByHeart officials said they recalled their products “in close collaboration” with the FDA, despite the fact that no previously unopened product tested positive for the illness-causing bacteria. The type of bacteria that produces the toxin is widespread in the environment and could come from sources other than the formula, company officials have said.
FDA inspectors have been to the company's infant formula production plants in Allerton, Iowa; and Portland, Oregon.
Families of babies treated for botulism after drinking ByHeart formula have sued the company. Lawsuits filed in federal courts allege that the formula they fed their children was defective and ByHeart was negligent in selling it. They seek financial payment for medical bills, emotional distress and other harm.
The FDA is investigating a rise in cases of infant botulism reported since August. “ByHeart brand formula is disproportionately represented among sick infants in this outbreak," the agency said.
Illnesses began between Aug. 9 and Nov. 13, federal officials said. Cases were reported in Arizona, California, Idaho, Illinois, Kentucky, Maine, Michigan, Minnesota, North Carolina, New Jersey, Oregon, Pennsylvania, Rhode Island, Texas and Washington.
Causes of infant botulism
Infant botulism typically affects fewer than 200 babies in the U.S. each year. As of Sept. 20, 133 cases had been reported in the U.S. according to the most recent Centers for Disease Control and Prevention records. There were 145 cases reported all of last year.
The infection is caused by a type of bacteria that produces a toxin in the large intestine. The bacterium is spread through hardy spores present in the environment that can cause serious illness, including paralysis.
Infants are particularly vulnerable to infection because their gut microbiomes are not developed enough to prevent the spores from germinating and producing the toxin. They can be sickened after exposure to the spores in dust, dirt or water or by eating contaminated honey.
Symptoms can take weeks to develop and can include poor feeding, loss of head control, drooping eyelids and a flat facial expression. Babies may feel “floppy” and can have problems swallowing or breathing.
Baby formula has previously been linked to sporadic cases of illness, but no known outbreaks of infant botulism tied to powdered formula have previously been confirmed, according to research studies.
Infant botulism treatment
The only treatment is known as BabyBIG, an IV medication made from the pooled blood plasma of adults immunized against botulism. California’s infant botulism program developed the product and is the sole source worldwide.
BabyBIG works to shorten hospital stays and decrease the severity of illness in babies with botulism. Because the infection can affect the ability to breathe, infants often need to be placed on ventilators.
All of the children in the ByHeart outbreak have received the medication, the CDC said. The treatment costs about $69,300 per child, California officials said.
Potential impact on U.S. formula supplies
There is little danger of infant formula shortages because ByHeart represents a small share of the market. That's far different from the crises in late 2021 and 2022, when four infants were sickened by a different germ after consuming formula made by Abbott Nutrition. Two of the babies died. No direct link was found between the Abbott products and the infections caused by a different germ, cronobacter sakazakii, but FDA officials closed the company's Michigan plant after contamination and other problems were detected.
Abbott recalled top brands of infant formula, triggering a nationwide shortage that lasted months.
In 2022, ByHeart recalled five batches of infant formula after a sample at the company's packaging plant tested positive for cronobacter sakazakii. In 2023, the FDA sent a warning letter to the company detailing “areas that still require corrective actions.” A ByHeart plant in Reading, Pennsylvania, was shut down in 2023 just before FDA inspectors found problems with mold, water leaks and insects, inspection documents show.
Reviewing infant formula ingredients
Federal health officials have vowed to overhaul the U.S. food supply and are taking a new look at infant formula.
Health Secretary Robert F. Kennedy Jr. has directed the FDA to review the nutrients and other ingredients in infant formula, which fills the bottles of millions of American babies.
The effort, dubbed “Operation Stork Speed,” is the first deep look at the ingredients since 1998.
FDA officials are reviewing comments from industry, health experts and public to decide next steps.
___





